|
|
SLEEK-EX |
|||||||||||||||||||
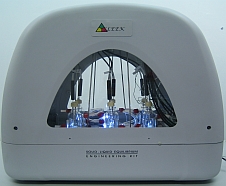
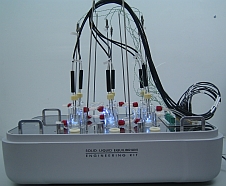
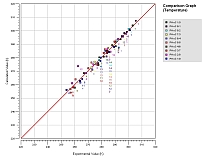
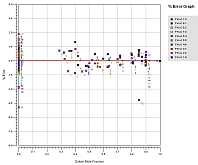
Solid-Liquid Equilibrium Engineering Kit - ExperimentsIntroductionWhy is SLE measurement important? Unlike Vapor Liquid Equilibrium (VLE) data, there are limited SLE data available in open literature. Very often the compound in question may even be a novel molecule that has no prior data at all. This is one of the most common setbacks in using SLE to design a crystallization-based separation system. SLE measurement is also important to gather information for process design and to develop an accurate model for crystallizer. We combine the strength of modeling of SLEEKTM and the strength of experiments of SLEEK-EX in solving the above critical design issue. For example, using the SLE modeling, synthesis and simulation abilities in SLEEKTM, and optimum operability conditions for the crystallizer can be identified with minimum thermodynamic information of the system. SLEEKTM helps the user identify operating conditions that give maximum recovery of a desired compound with a certain solvent or solvent mixture. Focused experiments can then be performed in parallel using SLEEK-EX to validate the SLE model and improve its accuracy.
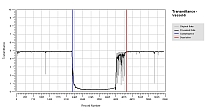
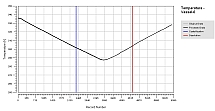
SLEEK-EX for Data MeasurementThere are two aspects of SLEEK-EX that are the key to its efficiency:
“Anyone looking to design crystallization unit operations should look seriously at the abilities of SLEEKTM to optimize overall plant design.” Dr. Timothy Nordahl How to OrderTo place an order, or for more information, please contact us by phone, or by email at sales(at)cwbtech.com. Reference Publications
If you would like to request a copy of any of the above papers, please click here.
|
|
||||||||||||||||||